The Impact of Upcoming Neurotechnologies on Alzheimer's Disease, Parkinson's Disease, and Spinal Cord Injury
DOI:
https://doi.org/10.58445/rars.51Keywords:
alzheimer's, spinal cord injury, parkinson's disease, neurotechnologiesAbstract
Neurological conditions are burdensome and have been extremely difficult to treat in the past. These conditions include Alzheimer's Disease, Parkinson's Disease, and spinal cord injury, which cause significant distress for patients and their loved ones. In recent years, advancements in science and biotechnology have allowed for current treatments and future applications for treating these diseases. Fortunately, these upcoming neurotechnologies provide us with the knowledge that there is a brighter future ahead for people who suffer from these conditions. Here, we discuss just some of these future breakthrough technologies, including neuroprosthetics, optogenetics, and pharmacologics, all of which have applications in improving the quality of life for people with Alzheimer’s Disease, Parkinson’s Disease, and traumatic spinal cord injury.
References
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 459–480 (2019).
Gooch, C. L., Pracht, E. & Borenstein, A. R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 81, 479–484 (2017).
Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers. Dement.
, 321–387 (2019).
Selkoe, D. J. Alzheimer Disease: Mechanistic Understanding Predicts Novel Therapies.
Ann. Intern. Med. 140, 627–638 (2004).
Querfurth, H. W. & LaFerla, F. M. Alzheimer’s disease. N. Engl. J. Med. 362, 329–344 (2010).
McLean, C. A. et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 46, 860–866 (1999).
Holtzman, D. M. et al. Tau: From research to clinical development. Alzheimers. Dement. 12, 1033–1039 (2016).
Hu, W. et al. Hyperphosphorylation determines both the spread and the morphology of tau pathology. Alzheimers. Dement. 12, 1066–1077 (2016).
Hoover, B. R. et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 (2010).
Spires-Jones, T. L. & Hyman, B. T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82, 756–771 (2014).
Dejanovic, B. et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 100, 1322–1336.e7 (2018).
DeVos, S. L. et al. Synaptic Tau Seeding Precedes Tau Pathology in Human Alzheimer’s Disease Brain. Front. Neurosci. 12, 267 (2018).
Ittner, A. & Ittner, L. M. Dendritic Tau in Alzheimer’s Disease. Neuron 99, 13–27 (2018).
Gibbons, G. S., Lee, V. M. Y. & Trojanowski, J. Q. Mechanisms of Cell-to-Cell Transmission of Pathological Tau: A Review. JAMA Neurol. 76, 101–108 (2019).
Soria Lopez, J. A., González, H. M. & Léger, G. C. Alzheimer’s disease. Handb. Clin. Neurol. 167, 231–255 (2019).
Dorsey, E. R. et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68, 384–386 (2007).
Cook Shukla, L. et al. Parkinson Disease Overview. in GeneReviews® (eds. Adam, M. P. et al.) (University of Washington, Seattle, 2004).
Goldman, S. M. et al. Concordance for Parkinson’s disease in twins: A 20-year update. Ann. Neurol. 85, 600–605 (2019).
LeWitt, P. A. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov. Disord. 30, 64–72 (2015).
Herrington, T. M., Cheng, J. J. & Eskandar, E. N. Mechanisms of deep brain stimulation. J. Neurophysiol. 115, 19–38 (2016).
McIntyre, C. C. & Anderson, R. W. Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J. Neurochem. 139 Suppl 1, 338–345 (2016).
Farokhniaee, A. & McIntyre, C. C. Theoretical principles of deep brain stimulation induced synaptic suppression. Brain Stimul. 12, 1402–1409 (2019).
Cagnan, H., Denison, T., McIntyre, C. & Brown, P. Publisher Correction: Emerging technologies for improved deep brain stimulation. Nat. Biotechnol. 37, 1237–1237 (2019).
Hachem, L. D., Ahuja, C. S. & Fehlings, M. G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord Med. 40, 665–675 (2017).
Alizadeh, A., Dyck, S. M. & Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 10, 282 (2019).
Anderson, K. D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 (2004).
Snoek, G. J., IJzerman, M. J., Hermens, H. J., Maxwell, D. & Biering-Sorensen, F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord 42, 526–532 (2004).
Collinger, J. L. et al. Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J. Rehabil. Res. Dev. 50, 145–160 (2013).
Kansaku, K. Neuroprosthetics in systems neuroscience and medicine. Sci. Rep. 11, 1–3 (2021).
Brown-Triolo, D. L., Roach, M. J., Nelson, K. & Triolo, R. J. Consumer perspectives on mobility: implications for neuroprosthesis design. J. Rehabil. Res. Dev. 39, 659–669 (2002).
Davis, J. A., Jr et al. Surgical technique for installing an eight-channel neuroprosthesis for standing. Clin. Orthop. Relat. Res. 237–252 (2001).
Fisher, L. E., Tyler, D. J., Anderson, J. S. & Triolo, R. J. Chronic stability and selectivity of four-contact spiral nerve-cuff electrodes in stimulating the human femoral nerve. J. Neural Eng. 6, 046010 (2009).
Dadia, T. & Greenbaum, D. Neuralink: The Ethical ‘Rithmatic of Reading and Writing to the Brain. AJOB Neurosci. 10, 187–189 (2019).
Fourneret, É. The Hybridization of the Human with Brain Implants: The Neuralink Project.
Camb. Q. Healthc. Ethics 29, 668–672 (2020).
Pisarchik, A. N., Maksimenko, V. A. & Hramov, A. E. From Novel Technology to Novel Applications: Comment on ‘An Integrated Brain-Machine Interface Platform With Thousands of Channels’ by Elon Musk and Neuralink. J. Med. Internet Res. 21, e16356 (2019).
Hennig, M. H., Hurwitz, C. & Sorbaro, M. Scaling Spike Detection and Sorting for Next-Generation Electrophysiology. Adv Neurobiol 22, 171–184 (2019).
Cutsuridis, V. Memory Prosthesis: Is It Time for a Deep Neuromimetic Computing Approach? Front. Neurosci. 13, 667 (2019).
Prox, J. et al. Toward living neuroprosthetics: developing a biological brain pacemaker as a living neuromodulatory implant for improving parkinsonian symptoms. J. Neural Eng. 18, (2021).
Kulshreshth, A., Anand, A. & Lakanpal, A. Neuralink- An Elon Musk Start-up Achieve symbiosis with Artificial Intelligence. in 2019 International Conference on Computing, Communication, and Intelligent Systems (ICCCIS) 105–109 (2019).
Musk, E. & Neuralink. An Integrated Brain-Machine Interface Platform With Thousands of Channels. J. Med. Internet Res. 21, e16194 (2019).
Fiani, B., Reardon, T., Ayres, B., Cline, D. & Sitto, S. R. An Examination of Prospective Uses and Future Directions of Neuralink: The Brain-Machine Interface. Cureus 13, e14192 (2021).
Balasubramanian, S. Elon Musk’s Neuralink Is Attempting To Make Brain-Machine Interfaces To Help Individuals With Paralysis. Forbes Magazine (2020).
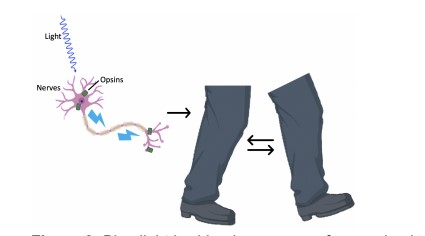
Ahmad, A., Ashraf, S. & Komai, S. Optogenetics applications for treating spinal cord injury. Asian Spine J. 9, 299–305 (2015).
Boyden, E. S. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol. Rep. 3, 11 (2011).
Adamantidis, A. R., Zhang, F., de Lecea, L. & Deisseroth, K. Optogenetics: opsins and optical interfaces in neuroscience. Cold Spring Harb. Protoc. 2014, 815–822 (2014).
Steinbeck, J. A. et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol. 33, 204–209 (2015).
Zimmer, M. B., Nantwi, K. & Goshgarian, H. G. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J. Spinal Cord Med. 30, 319–330 (2007).
Onders, R. P. et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg. Endosc. 23, 1433–1440 (2009).
Wang, K.-W., Ye, X.-L., Huang, T., Yang, X.-F. & Zou, L.-Y. Optogenetics-induced activation of glutamate receptors improves memory function in mice with Alzheimer’s disease. Neural Regeneration Res. 14, 2147–2155 (2019).
By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 63, 2227–2246 (2015).
Ferrero, J. et al. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers. Dement. 2, 169–176 (2016).
Mintun, M. A. et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 384, 1691–1704 (2021).
Koller, W. C. & Rueda, M. G. Mechanism of action of dopaminergic agents in Parkinson’s disease. Neurology 50, S11–S14 (1998).
Cruz, M. P. Xadago (Safinamide): A Monoamine Oxidase B Inhibitor for the Adjunct Treatment of Motor Symptoms in Parkinson’s Disease. P T 42, 622–637 (2017).
Fénelon, G., Mahieux, F., Huon, R. & Ziégler, M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 123 ( Pt 4), 733–745 (2000).
Schrag, A., Ben-Shlomo, Y. & Quinn, N. How common are complications of Parkinson’s disease? J. Neurol. 249, 419–423 (2002).
Goetz, C. G., Fan, W., Leurgans, S., Bernard, B. & Stebbins, G. T. The Malignant Course of ‘Benign Hallucinations’ in Parkinson Disease. Arch. Neurol. 63, 713–716 (2006).
Lang, A. E. et al. Trial of Cinpanemab in Early Parkinson’s Disease. N. Engl. J. Med. 387, 408–420 (2022).
Pagano, G. et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 387, 421–432 (2022).
Downloads
Posted
Versions
- 2022-12-20 (2)
- 2022-11-02 (1)
Categories
License
Copyright (c) 2022 Zeeshan Haq

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
